The lifetime of fuel cells is the main obstacle to their large-scale deployment, particularly in the transport sector. Scientists have shown that some localized defects in the electrodes can propagate to the fuel cell membrane. Published in the Journal of Power Sources, this work is based in particular on ultra-sophisticated cells that make it possible to monitor the electrochemical state of fuel cells in detail, combined with fine analysis of the materials.
Capable of producing energy from hydrogen and oxygen from the air, fuel cells are valuable tools for the energy transition. Ideally, they would be used in heavy transport applications, such as trains and trucks, but this would require commercial fuel cell systems to have a lifetime of at least 20 000 hours, which is not yet the case. One way to improve this parameter is to better understand the defects that can occur in the main components of a fuel cell core, which are often made of fragile materials that are difficult to shape. The thickness of the ionomer membrane, which separates the electrodes and acts as an electrolyte, is less than 20 µm, i.e. one-fifth that of a human hair, while the electrodes take the form of platinum nanoparticles deposited on submicrometre carbon particles. The work, which is the result of a thesis by Salah Touhami and the “Hydrogen and Electrochemical Systems” team at Lemta, in collaboration with LEPMI and LITEN, has shown that defects in the fuel cell are capable of propagating within the device, with a detrimental impact on its proper functioning. The results are published in the Journal of Power Sources.
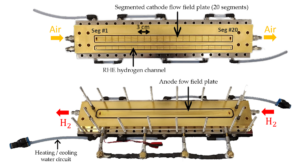
This phenomenon was observed using segmented and instrumented cells from LEMTA. With conventional cells, only the voltage, current and nature of the gases are measured, and only at the input and output of the device. The LEMTA cells, a rare piece of equipment even on a global scale, offer local measurements at many other points and provide information on the majority of electrochemical parameters, such as impedance or the capacity of hydrogen to cross the membrane. The research teams have studied the impact of defects, such as the local absence of an anodic catalytic layer, on the health of the fuel cell. These weaknesses allow oxygen from the air and hydrogen to pass through the membrane. Together they form hydrogen peroxide and free radicals, highly reactive compounds that significantly degrade the membrane, allowing more gas to pass through and so on. The researchers also showed that the defects propagated mainly in the direction of the hydrogen flow and, at least initially, much less in the direction of the air flow. The scientists are now exploring the details of the defect propagation mechanisms, within the framework of the PEPR Decarbonated Hydrogen (Priority Research Programmes and Equipment), in order to contribute to improving the lifetime of fuel cells.
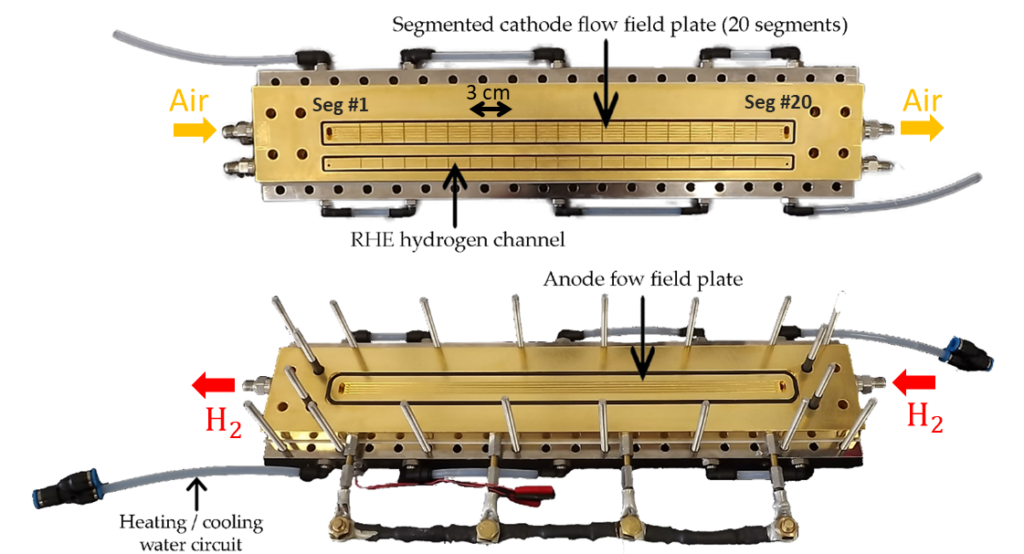
Segmented and instrumented cells at LEMTA, with the cathode and the air and hydrogen supply channels for reference electrodes at the top, and the anode and hydrogen supply channel at the bottom. © Salah Touhami
References
Anode defects’ propagation in polymer electrolyte membrane fuel cells.
Salah Touhami, Marie Crouillere, Julia Mainka, Jérôme Dillet, Christine Nayoze-Coynel, Corine Bas, Laetitia Dubau, Assma El Kaddouri, Florence Dubelley, Fabrice Micoud, Marian Chatenet, Yann Bultel, Olivier Lottin.
Journal of Power Sources 520, 2022.
https://doi.org/10.1016/j.jpowsour.2021.230880
Article source
Website Institute of Engineering Sciences and Systems (INSIS) of the CNRS